Department Personnel
Associate Professor Antonios Armaou | Research
Mathematical modeling of HIV infection
HIV infection has been subject of enormous research studies and substantial resources have been dedicated to find a cure for this disease. Mathematical models of HIV dynamics are helpful in predicting the progression of the infection and hence investigating the effects of treatments on its dynamics. Our group currently focuses on developing advanced models in the
Extracellular model: The two most important classes of commonly administrated drugs are reverse transcription inhibitor, RTI, and protease inhibitor, PI. However, mutation plays an important role in reducing the affectivity of the drugs. During replication of virus, mutation causes emergence of new strands of virus which might be resistant to these drugs. We assumed two distinctive populations of virus, each being resistant to one class of drugs, i.e., resistant to RTI and resistant to PI. The figures below show the interactions among virus (V), healthy T-cell (T), latently infected T-cell (L), actively infected T-cell (I), non infectious virus (VN), resistant to RTI virus (VmR), resistant to PI virus (VmP), and T-cells infected with resistant virus.
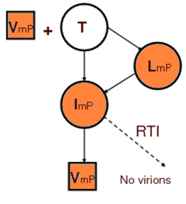
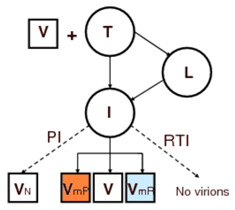
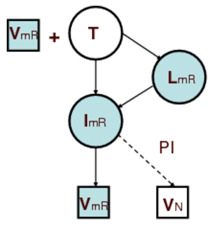
The focus of this work is the early stage of infection when the virus population is very small and random fluctuations have important effects on the dynamics of the system. In other words, infection eradication is still possible at this stage and its probability increases with administration of RTI and PI. Consequently, we employed stochastic simulation to quantify this probability and also sensitivity analysis results regarding to drug type and efficacy were presented. For in depth discussions see S. Khalili and A. Armaou, Chem. Eng. Sci., 63 (5), Mar 2008.
The current HIV drugs have toxic effects on patients. Currently a high dosage, short period treatment regime is prescribed for patients at early stage of infection. However, it is reported that 20% of the patient receiving postexposure prophylaxis discontinued treatment because of developing symptoms (nausea, fatigue, vomiting, etc.). Thus, it is important to schedule the optimal medication strategy for patients in the primary stage of HIV infection. We sought optimal treatment strategies which maximize the probability of infection eradication and simultaneously minimize the dosage of medication over time. This objective is mathematically formulated as a dynamic optimization problem. For results and discussions see S. Khalili and A. Armaou, Chem. Eng. Sci., 63 (17), Sep 2008.
Intracellular model: In the current extracellular model, the values of the parameters that describe the infection dynamics are obtained by curve fitting of, usually sparsely available, hospital data. To increase the reliability of the predictions, developing more accurate models considering infection dynamics at both extra and intracellular levels is necessary.
The interaction dynamics of intracellular components are extremely complicated and on the other hand, quantitative information from experimental studies does not include all parts of the cycle. These obstacles make developing intracellular mathematical models of HIV very difficult. The figure below shows the intracellular events of the infection cycle. After successful binding between virus and the host cell, the virus contents enter the cytoplasm of the target cell. The next step is the reverse transcription of viral RNA to DNA followed by transfer of the produced DNA to the host nucleus and integration of it to the host genome. Once integrated, the provirus is replicated along with the host cell DNA. The HIV transcript undergoes a series of post-transcriptional splicing events. This results in the generation of over 20 different mRNAs. Finally, necessary viral contents and proteins gather at the surface of the host and new virions bud from the membrane.
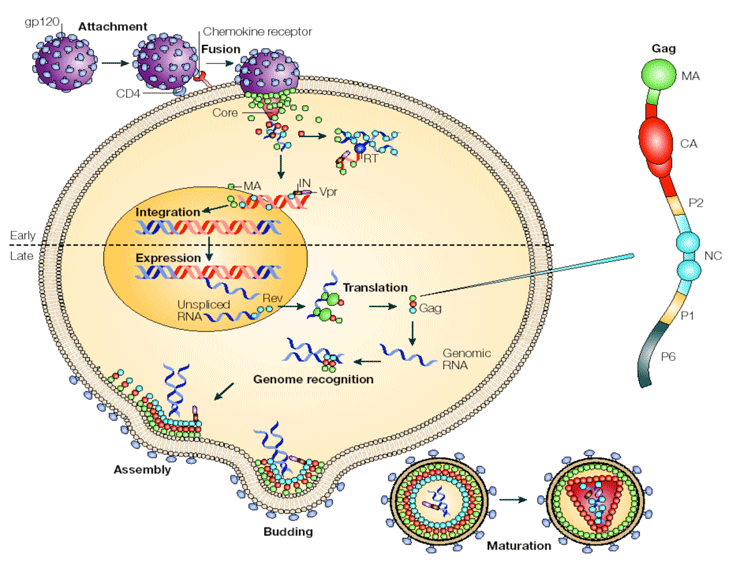
Nature Reviews: Microbiology, 3(8), pp 643-655, 2005.
In this work we propose an intracellular model which covers the complete infection cycle yet is detailed enough that can provide a good insight into the HIV infection cycle. Furthermore, this detailed model provides information about dynamics of viral components which can improve drug administration scheduling.
Ultimately, this model will be linked to the extracellular model by coarsening the intracellular events and generate a multiscale model of HIV infection. The time scale of intracellular events inside the infected host is a few orders of magnitude smaller than the time scale of cell population interactions at the extracellular level. The developed model will enhance the understanding of infection cycle and will be useful in predicting the overall infection dynamics while considering the intracellular infection events.
Page created on 02/23/11, Top of page


