Research Topics
Catalysis by doped oxides: M-CeO2 systems
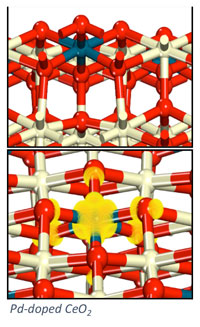
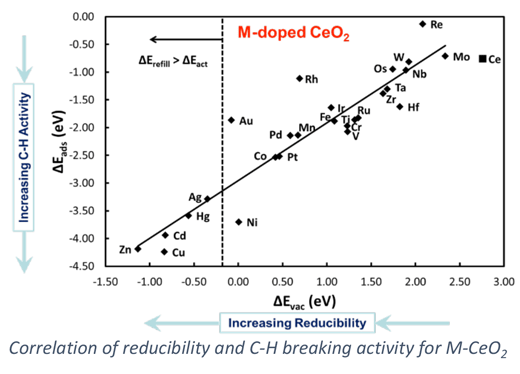
Doped oxides (M1-M2Ox) can offer tunable properties for optimization of catalytic performance. For example, an “active” and expense metal can be doped into the surface of a high surface area support to provide greater oxidation activity, allowing for improved performance and ideal dispersion of the expensive material. Our work on doped oxides has concentrated on the design of metal-doped ceria (M-CeO2) materials for hydrocarbon oxidation and desulfurization. Electronic structure methods, specifically density functional theory, are used to examine surface reaction processes involved in hydrocarbon conversion. Our goal is to predictively design stable and active doped metal oxides for efficient oxidation catalysis.
Our work on doped oxides has relevance to a number of catalytic applications include hydrocarbon reforming for H2 or syngas production, gasifier effluent clean-up, catalytic combustion, and partial oxidation for alkane conversion to higher value chemicals. Our computational work emphasizes use of DFT+U methods and the evaluation of their reliability to predict catalytic properties as well as the integration of reactive force-field methods to examine reactivity in the complex structures that occur under reaction environments.
We collaborate with a number of other faculty in research area of doped metal-oxides, including Kerry Dooley (Louisiana State U.), Matthias Batzill (U. of South Florida), Adri van Duin (Penn State Mech E), and Jason Weaver (U. of Florida).