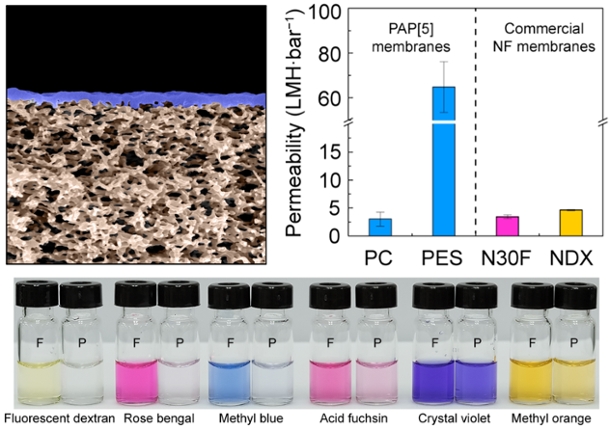
Scanning electron microscope image of artificial water channel based membranes, which have an order of magnitude higher water productivity compared to commercial membranes (N30F and NDX). This image is adapted from the original article (Nature Communications 9, Article number: 2294 (2018)) and is licensed under a Creative Commons Attribution 4.0 International License.
Penn State leading innovation in nascent field of artificial water channels
September 19, 2018
UNIVERSITY PARK, Pa. – The Department of Chemical Engineering’s cutting-edge work in the nascent field of artificial water channels was the subject of a recent Faraday Discussions conference held by the Royal Society of Chemistry and a breakthrough paper in the journal Nature Communications.
Manish Kumar, associate professor of chemical engineering, biomedical engineering, and civil and environmental engineering, was the Deputy Chair of the 2018 Artificial Water Channels Faraday Discussions, held in Glasgow, Scotland on June 25-27. The event was attended by engineers and scientists from around the world focusing on creating protein-mimicking supramolecular channels that can selectivity transport water at the rate of billions of water molecules per second while excluding dissolved substances such as salts or contaminants. The Royal Society of Chemistry initiated the Faraday Discussions in 1907 as a way to encourage the development of emerging fields of study, such as artificial water channels.
Kumar noted that research into artificial water channels began around 2011 or 2012, so it is a good topic for such an event. He also added that in this short time, Penn State has emerged as a leader in this young field with a publication in 2015 in the journal Proceedings of the National Academy of Sciences demonstrating that a class of artificial channels, known as peptide-appended-pillar[5]arene (PAP) channels can approach the water transport efficiency of biological channels within an order of magnitude. Penn State’s water channel research is supported by a five-year National Science Foundation grant as well as another grant from the US Army.
The best way to describe artificial water channels, Kumar said, are as synthetic analogs for biological water channel proteins, aquaporins or as mimics of carbon nanotubes with angstrom scale pores. In most living systems, aquaporins transport water at rates of around a billion to 10 billion water molecules per second while excluding everything else. Such rates are not currently possible in any man-made structure with appreciable selectivity for dissolved substances.
“We want to study these biological water channels because they are so efficient at transporting water,” Kumar said. “They could make for really good membranes if we could take them from biological substances and successfully put them into synthetic materials.”
Kumar said that it is very difficult to make membranes by using biological water channels because these proteins are generally considered not stable for engineering applications. So, Kumar and his team are working on making more stable artificial analogs of the biological water channels. Adding to the challenges is the fact that mimicking the function of the complex superstructures of these proteins is difficult.
It is worth the effort to overcome these obstacles because there are plenty of real world applications for artificial water channels. Depending on the pore size of these water channels, they could be used to make membranes for pharmaceutical separations and other biomedical applications. If they can be made tight enough, the fast transport of water through the channels can be used for advanced desalination techniques for water treatment.
In a recent 2018 publication, Kumar and coworkers, including Dr. Robert Hickey, assistant professor of materials science and engineering, created the first macroscale membranes ever made using artificial channels. These channels that can be possibly used to separate small molecules such as antibiotics from fermentation media where it is produced has a productivity that is an order of magnitude higher than current commercial membranes while maintaining the same selectivity. This work was published in the journal Nature Communications.
To help solve the challenges of creating even more efficient artificial water channels, the focus of the Faraday Discussion on water channels was the chemistry, physics and material sciences breakthroughs in the rapidly evolving field of artificial water channels. In addition, there was discussion on how to uncover some of the novel water-channel interactions that might parallel those in biomolecular systems.
The format of a Faraday Discussion includes having presenters write and submit a paper before the event to be reviewed by attendees. Then there is a short presentation to begin the event, followed by 45 minutes of discussion. The 45-minute discussion features individuals who either agree or disagree with the paper, and often these attendees present their own data for discussion. The entire Faraday Discussion is recorded on paper and after it is reviewed by the discussion committee, the entire discussion is published.
“You get to see how many [academics] agree with a certain point of view versus disagree, and it’s a civil discussion. In this day and age, there is so much controversy about what’s true and factual, [so] this [format] is good,” Kumar said. “This is a very powerful meeting, which has given birth to many new fields in chemistry.”
At the end of the Faraday Discussion Conference, Kumar said there is a dinner, and an unusual and fun ceremony called the Loving Cup Ceremony. A cup from the 1400s is filled with port wine and is passed around so participants each make a statement honoring two people that helped support the Faraday discussion before taking a sip, and then passing it on to the next person.
“It's almost like a religious ceremony,” Kumar said. “It’s the first time I've ever seen something like this done at a conference.”
Along with Kumar and Song, Robert Hickey, assistant professor of materials science and engineering, attended the conference. Chao Lang, a chemical engineering and materials science post-doctoral scholar, did not attend but his work was among those papers presented.



